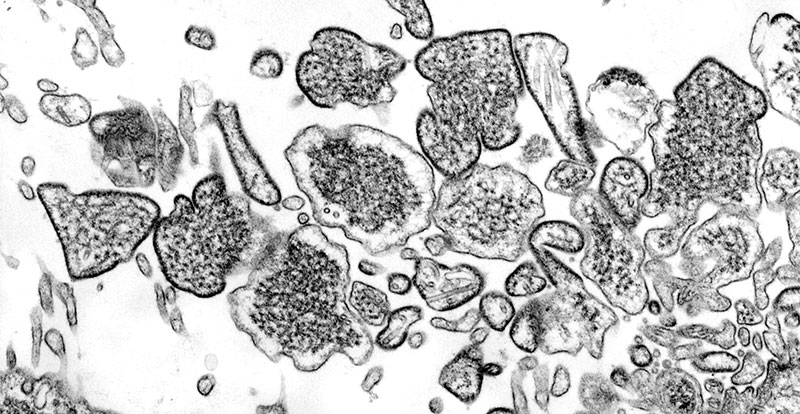
KUALA LUMPUR – Human trials for an experimental vaccine against the deadly Nipah virus, which was first identified in Malaysia 25 years ago, have started at Oxford University.
The first participants received doses of the vaccine over the last week, said the university’s Pandemic Sciences Institute.
It added that the trial vaccine is based on the same technology as the one used in Covid-19 shots developed by AstraZeneca and Serum Institute of India, reported Reuters.
The 51-patient trial will examine immune responses of people aged 18 to 55 to the vaccine, and more will be carried out later in a Nipah-affected country.
“Nipah has epidemic potential, with its fruit bat hosts found in areas home to over two billion people.
“This trial is a step forward in efforts to build a suite of tools to protect against this killer virus,” Dr In-Kyu Yoon, an executive at the Coalition for Epidemic Preparedness Innovations (Cepi), was quoted as saying.
The trial is led by Oxford Vaccine Group and funded by Cepi.
In 2022, Pharma company Moderna started an early-stage clinical trial of a Nipah vaccine, which was developed with the National Institute of Allergy and Infectious Diseases in the US.
In September last year, Kerala in India experienced its fourth Nipah outbreak in five years.
The World Health Organisation has estimated Nipah’s fatality rate at 40% to 75%. – January 11, 2024